Introduction of gold plating
1.Gold is a golden precious metal that is malleable and easy to polish.
2.Gold has good chemical stability, insoluble in ordinary acids, only soluble in aqua regia
3.Gold coating has strong corrosion resistance and good resistance to discoloration
4.Gold plating has a variety of colors, also used for expensive decorative coatings
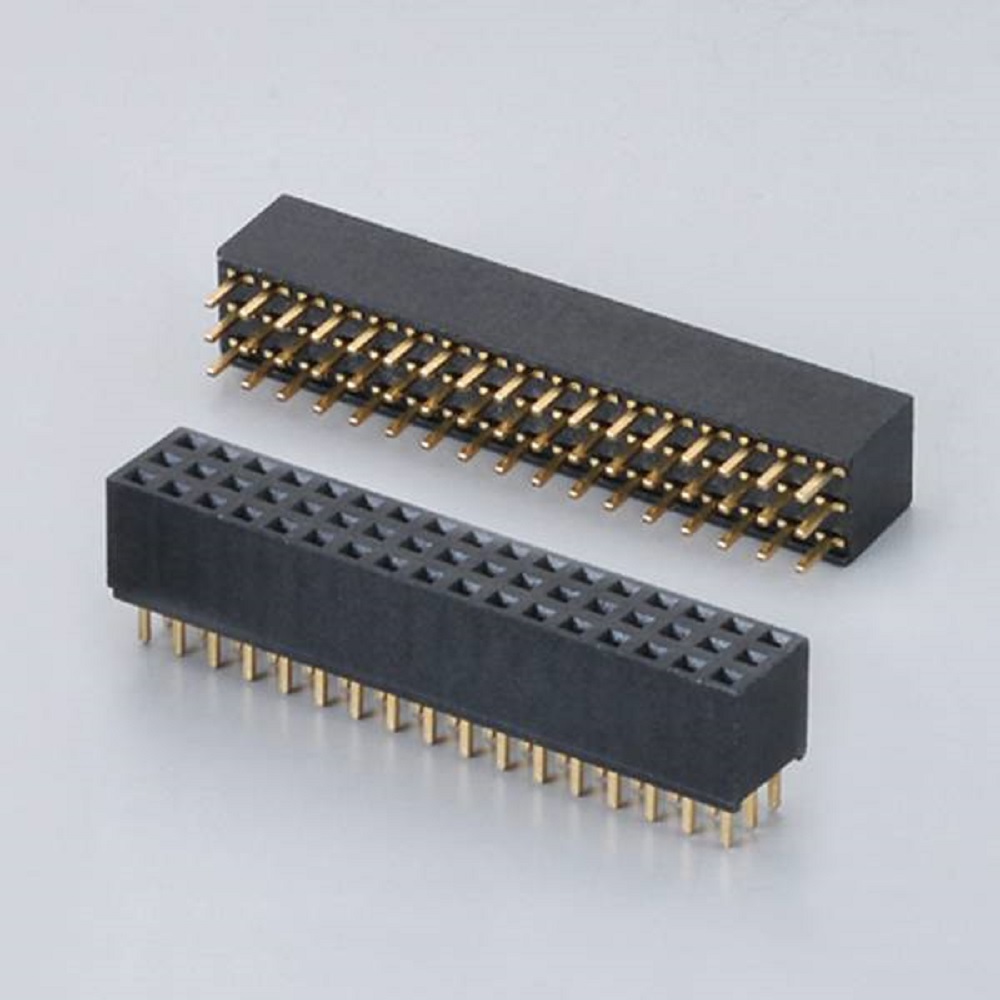
FEMALE HEADER PITCH:2.0MM(.047″) TRIPLE ROW STRAIGHT 180°
5.Gold has low contact resistance and good conductivity, and is often used in sliding contact situations.
6.Gold plating is easy to welding and has good temperature resistance,certain wear-resisting performance, but take care, it is not thick gold more easy to welding, on the contrary, the thickness of gold layer 3-5 ų ° welding effect is best.
7.The addition of copper to gold has little effect on the hardness, but the addition of 10% nickel has great effect on the hardness. Besides, au-NI alloy has high stability.
8.Poor air tightness of gold, bottom gold will have diffusion phenomenon.Generally with nickel base, leave to prevent gold bottom diffusion
9.Gold has a low melting point and is easily soluble in tin during nickel welding, resulting in the formation of AU-SN compounds and the formation of gold brittleness
10. Anticorrosive ability of the original copper alloy plating on nickel ų 50 ° is very good, but as long as in nickel – plating a layer of a text, corrosion resistant ability is very poor.The reason is that the potential difference between gold and nickel is very large, which causes the accelerated corrosion reaction of Galliani. The salt spray experiment proves that this theory is correct. Originally, nickel without thin gold plating can last 72 hours, while nickel with thin gold plating cannot last 48 hours.
Introduction of tin electroplating
1.Tin has a silver-white appearance.
2.Tin is corrosion-resistant, color-resistant, non-toxic, easy to weld and ductile
3.Tin coating has high chemical stability
4.Electrical conductivity of tin coating is good, easy to weld, and often in place of silver tin
5.in coating has the phenomenon of tin fever, but not with bismuth, antimony alloy\
6.Tin coating at high temperature, wet, sealed conditions will produce tin whiskers.
7.The melting point of tin-lead alloy is lower than pure lead and pure tin, its porosity and weldability is better than the single metal, in pure tin as long as the addition of 2-3% lead, it is not easy to produce tin whisker, so tin-lead alloy coating is the most important solderable coating in electronic components, can replace the silver coating within a certain range.
8.It is Often used for welding materials and can also be used for larger positive force contact coating.
Post time: Jul-22-2020